About IncreCare
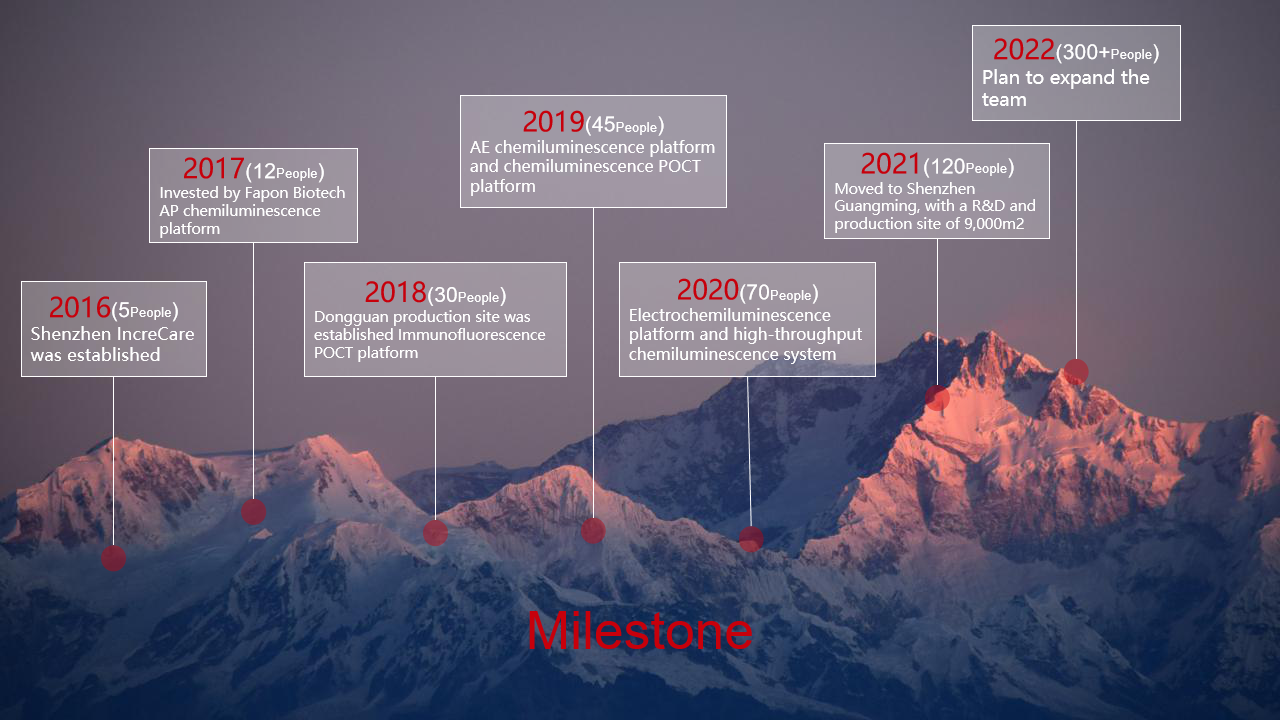
Shenzhen IncreCare, established in December 2016, is a national high-tech in vitro diagnostic device provider. It has developed into one of the strongest comprehensive technical strength in China providing open-system devices and solutions. The products and professional technical services have won the trust and support of more than dozens of in vitro diagnostic companies across the world。
The company has achieved ISO 13485 certification for medical devices quality management system, ISO 9001 certification for international quality management, CE certification, ISO 14001:2015 certification for environmental management system and medical device production license
Shenzhen IncreCare attaches great importance to independent innovation. Currently, the company has more than 140 employees. Among them, 50% are engaged in full-time R&D, of which 20 core team members have more than 10 years of product development and production experience in well-known medical enterprises. The company will continue to grow and is expected to have 200 or more employees by mid 2022
Since its establishment, IncreCare has focused on the development of open-system chemiluminescence analyzer. The company has now successfully launched a variety of products including chemiluminescence immunoassay analyzer, immunofluorescence analyzer and other in vitro diagnostic products. In the past 5 years, the company has applied for nearly 200 patents, including more than 60 international patents.

Milestone